Patient-Reported Outcome (PRO) Instrument
A Patient-Reported Outcome (PRO) Instrument is a clinical outcome assessment instrument that is based on self-reported perception-based information (from a patient or clinical study participant).
- AKA: Self-Report COA.
- Context:
- output: PRO Score that produces PRO values)
- It can (typically) be composed of a PRO Questionnaire and a PRO Questionnaire Scoring Method.
- It can (typically) be instantiated into a PRO-Study instance.
- It can (often) be a Language-Specific PRO, such as an English Language PRO.
- It can range from being a Disease-Specific PRO, a Condition-Specific PRO, or a Generic PRO.
- It can range from being a Paper-based PRO to being an Electronic-based PRO.
- It can range from being a Disease/Condition-Specific PRO to being a Non-Disease/Condition-Specific PRO.
- It can range from being a Scheduled PRO to being an Unscheduled PRO.
- It can range from being a Time-Restricted PRO to being a Time-Unrestricted PRO.
- It can be associated with a PRO Compliance Measure.
- It can be associated with a PRO Filling-In Session, that can result in a filled-in PRO.
- It can be associated with a PRO Creation Best Practice.
- …
- Example(s):
- Disease-Specific PRO Measures, such as:
- Cancer PROs, such as:
- Migraines PROs, such as: Migraine Physical Function Impact Diary (MPFID).
- Psoriasis PROs, such as: Psoriasis Symptom Inventory (PSI).
- Respiratory Diseases PROs, such as: St George’s Respiratory Questionnaire (SGRQ).
- Mental Health PRO, such as:
- Patient Health Questionnaire (PHQ-9) - A common screening tool for depression.
- Generic PRO Measures, such as:
- EuroQol Group's 5-Dimension Health Questionnaire (EQ-5D) - A standardized measure of health status.
- Health-Related Quality of Life (HRQL) PRO Measures, such as:
- a Ratings-Scale PRO (that uses a rating scale to assess a patient’s symptom severity or frequency), such as:
- Counts-of-Events PRO that Tracks the frequency of a particular event, such as episodes of headache.
- …
- Disease-Specific PRO Measures, such as:
- Counter-Example(s):
- See: PROMIS Organization, Patient Intervention Outcome, Electronic Clinical Outcome Assessment (eCOA) System, Decentralized Clinical Trial, Patient-Reported Outcome-Based Performance Measure (PRO-PM).
References
2021a
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Patient-reported_outcome Retrieved:2021-12-12.
- A patient-reported outcome (PRO) is a health outcome directly reported by the patient who experienced it. It stands in contrast to an outcome reported by someone else, such as a physician-reported outcome, a nurse-reported outcome, and so on. PRO methods, such as questionnaires, are used in clinical trials or other clinical settings, to help better understand a treatment's efficacy or effectiveness. The use of digitized PROs, or electronic patient-reported outcomes (ePROs), is on the rise in today's health research setting.
2021b
- (NCBI, 2021) ⇒ https://www.ncbi.nlm.nih.gov/books/NBK338448/def-item/glossary.patientreported-outcome/
- QUOTE: A type of clinical outcome assessment. A measurement based on a report that comes directly from the patient (i.e., study subject) about the status of a patient’s health condition without amendment or interpretation of the patient’s response by a clinician or anyone else. A PRO can be measured by self-report or by interview provided that the interviewer records only the patient’s response. Symptoms or other unobservable concepts known only to the patient can only be measured by PRO measures. PROs can also assess the patient perspective on functioning or activities that may also be observable by others. PRO measures include:
- Rating scales (e.g., numeric rating scale of pain intensity or Minnesota Living with Heart Failure Questionnaire for assessing heart failure)
- Counts of events (e.g., patient-completed log of emesis episodes or micturition episodes)
- QUOTE: A type of clinical outcome assessment. A measurement based on a report that comes directly from the patient (i.e., study subject) about the status of a patient’s health condition without amendment or interpretation of the patient’s response by a clinician or anyone else. A PRO can be measured by self-report or by interview provided that the interviewer records only the patient’s response. Symptoms or other unobservable concepts known only to the patient can only be measured by PRO measures. PROs can also assess the patient perspective on functioning or activities that may also be observable by others. PRO measures include:
2021c
- (Efficace et al., 2021) ⇒ Fabio Efficace, Johannes M Giesinger, David Cella, Francesco Cottone, Francesco Sparano, Marco Vignetti, and Neil K Aaronson. (2021). “Investigating Trends in the Quality of Reporting of Patient-Reported Outcomes in Oncology Over Time: Analysis of 631 Randomized Controlled Trials Published Between 2004 and 2019.” In: Value in Health. doi:https://doi.org/10.1016/j.jval.2021.06.003
- QUOTE: Patient-reported outcomes (PROs) are increasingly included as endpoints in randomized controlled trials (RCTs). Previous studies have highlighted poor standards of PRO reporting from cancer RCTs, which may compromise the understanding and impact of PRO study findings.
2020
- (Cochrane PRO-MG, 2020) ⇒ https://handbook-5-1.cochrane.org/chapter_17/17_1_what_are_patient_reported_outcomes.htm
- QUOTE: Patient-reported outcomes (PROs) are any reports coming directly from patients about how they function or feel in relation to a health condition and its therapy, without interpretation of the patient’s responses by a clinician, or anyone else. PROs include any treatment or outcome evaluation obtained directly from patients through interviews, self-completed questionnaires, diaries or other data collection tools such as hand-held devices and web-based forms (US Food and Drug Administration 2006). Proxy reports from caregivers, health professionals, or parents and guardians (necessary in some conditions such as advanced cancer and cognitive impairment) cannot be considered PROs and should be considered as a separate category of outcomes.
PROs provide patients’ perspective on treatment benefit; directly measure treatment benefit beyond survival, disease, and physiologic markers; and are often the outcomes of greatest importance to patients. Reports from patients may include the signs and symptoms reported in diaries, the evaluation of sensations (most commonly classified as symptoms), reports of behaviours and abilities (most commonly classified as functional status), general perceptions or feelings of well-being, and other reports including satisfaction with treatment, general or health-related quality of life, and adherence to treatments. Reports may also include adverse or side effects (see Chapter 14).
PROs are sometimes used as primary outcomes in clinical trials, particularly when no surrogate measure of direct benefit is available to capture the patient’s well-being. More often, PROs complement primary outcomes such as survival, disease indicators, clinician ratings and physiologic or laboratory-based measures. Figure 17.1.a shows those outcomes that are considered most often as important to patients within a classification of all outcomes.
PROs may be collected using a measure (or instrument) that is disease-specific, condition-specific or generic. Disease-specific measures describe severity, symptoms, or functional limitations specific to a particular disease state, condition or diagnostic grouping (e.g. arthritis or diabetes). Condition-specific measures describe patient symptoms or experiences related to a specific condition or problem (e.g. low-back pain) or related to particular interventions or treatments (e.g. knee-replacement or coronary artery bypass graft surgery). Generic measures are designed for use with any illness group or population sample.
- QUOTE: Patient-reported outcomes (PROs) are any reports coming directly from patients about how they function or feel in relation to a health condition and its therapy, without interpretation of the patient’s responses by a clinician, or anyone else. PROs include any treatment or outcome evaluation obtained directly from patients through interviews, self-completed questionnaires, diaries or other data collection tools such as hand-held devices and web-based forms (US Food and Drug Administration 2006). Proxy reports from caregivers, health professionals, or parents and guardians (necessary in some conditions such as advanced cancer and cognitive impairment) cannot be considered PROs and should be considered as a separate category of outcomes.
2018
- (FDA, 2021) ⇒ https://www.fda.gov/drugs/development-approval-process-drugs/patient-focused-drug-development-glossary Content current as of: 06/08/2018.
- QUOTE: Patient-reported outcome (PRO): A measurement based on a report that comes directly from the patient (i.e., study subject) about the status of a patient's health condition without interpretation of the patient's response by a clinician or anyone else. A PRO can be measured by self-report or by interview, provided that the interviewer records only the patient's response. Symptoms or other unobservable concepts known only to the patient (e.g., pain severity or nausea) can only be measured by PRO measures. PROs can also assess the patient perspective on functioning or activities that may also be observable by others (...)
2017
- (Bresnick) ⇒ Jennifer Bresnick (2017). “Why Patient-Reported Outcomes Data is Key to Healthcare Quality". In: HealthITAnalytics - Xtelligent Healthcare Media.
- QUOTE: ... Achieving this goal requires the health system to learn how to collect raw patient-reported outcomes in a standardized manner (PROMs) and then how to translate that standardized data into a performance measure (PRO-PMs) that captures the results most important for improving long-term wellness. ...
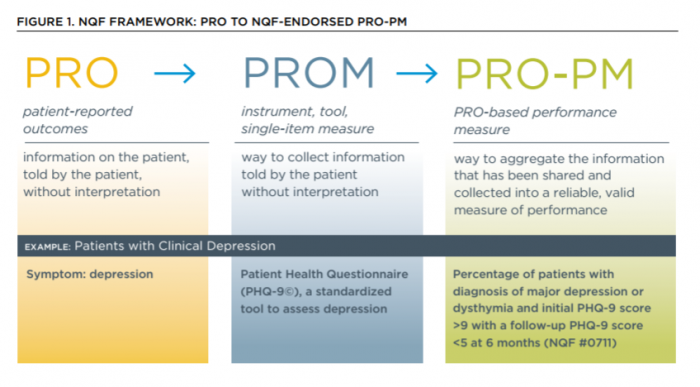
- QUOTE: ... Achieving this goal requires the health system to learn how to collect raw patient-reported outcomes in a standardized manner (PROMs) and then how to translate that standardized data into a performance measure (PRO-PMs) that captures the results most important for improving long-term wellness. ...
2012
- (Arthurs et al., 2012) ⇒ Erin Arthurs, Russell J. Steele, Marie Hudson, Murray Baron, Brett D. Thombs, and (CSRG) Canadian Scleroderma Research Group. (2012). “Are Scores on English and French Versions of the PHQ-9 Comparable? An Assessment of Differential Item Functioning.” In: PloS One, 7(12).
- QUOTE: Health-related patient-reported outcomes (HR-PROs) assess patient health based on patient perspectives. HR-PROs may reflect complex constructs, such as health-related quality of life (HRQL), or narrower constructs, such as pain, fatigue or depressive symptoms [1]–[3]. Assessment of HR-PROs has been emphasized in recent years, especially among patients with chronic diseases [4], and this has been reflected in initiatives aimed at improving measurement quality and operationalization in research and clinical practice, such as the PROMIS initiative [5] and OMERACT in rheumatology [6]. Recently, the COSMIN checklist (Consensus based Standards for the selection of health status Measurement INstruments) was developed to establish general criteria for assessing the methodological quality of studies that evaluate measurement properties of HR-PROs [7].
In recent years, international collaboration, and, thus, interest in HR-PRO research in the context of multinational studies, has grown [8]. HR-PROs are increasingly translated and used in diverse linguistic and cultural settings [9]. As described in the COSMIN checklist [7], the cross-linguistic or cross-cultural equivalence of HR-PRO measures is an important consideration for instruments that will be administered in more than one language or for comparisons of results across linguistic or cultural settings.
- QUOTE: Health-related patient-reported outcomes (HR-PROs) assess patient health based on patient perspectives. HR-PROs may reflect complex constructs, such as health-related quality of life (HRQL), or narrower constructs, such as pain, fatigue or depressive symptoms [1]–[3]. Assessment of HR-PROs has been emphasized in recent years, especially among patients with chronic diseases [4], and this has been reflected in initiatives aimed at improving measurement quality and operationalization in research and clinical practice, such as the PROMIS initiative [5] and OMERACT in rheumatology [6]. Recently, the COSMIN checklist (Consensus based Standards for the selection of health status Measurement INstruments) was developed to establish general criteria for assessing the methodological quality of studies that evaluate measurement properties of HR-PROs [7].
2009
- (FDA, 2009) ⇒ U.S. Department of Health and Human Services Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Devices and Radiological Health (CDRH) (2009) "Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims (Guidance for Industry)". In: FDA Guidance Document, Docket Number: FDA-2006-D-0362.
- QUOTE: A PRO is any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else. The outcome can be measured in absolute terms (e.g., severity of a symptom, sign, or state of a disease) or as a change from a previous measure. In clinical trials, a PRO instrument can be used to measure the effect of a medical intervention on one or more concepts (i.e., the thing being measured, such as a symptom or group of symptoms, effects on a particular function or group of functions, or a group of symptoms or functions shown to measure the severity of a health condition).
Generally, findings measured by a well-defined and reliable PRO instrument in appropriately designed investigations can be used to support a claim in medical product labeling if the claim is consistent with the instrument’s documented measurement capability. The amount and kind of evidence that should be provided to the FDA is the same as for any other labeling claim based on other data. Use of a PRO instrument is advised when measuring a concept best known by the patient or best measured from the patient perspective. A PRO instrument, like physician-based instruments, should be shown to measure the concept it is intended to measure, and the FDA will review the evidence that a particular PRO instrument measures the concept claimed. The concepts measured by PRO instruments that are most often used in support of labeling claims refer to a patient’s symptoms, signs, or an aspect of functioning directly related to disease status. PRO measures often represent the effect of disease (e.g., heart failure or asthma) on health and functioning from the patient perspective(...)
For the FDA to provide useful early input, sponsors should provide their labeling goals, a hypothesized PRO instrument conceptual framework, and the relationship of the PRO endpoints to other clinical trial endpoints in preliminary endpoint models for the planned confirmatory trials.
If the measurement goal is to support a complex, multidomain concept, PRO instruments that measure a simple concept may not be adequate to substantiate the complex claim.
- QUOTE: A PRO is any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else. The outcome can be measured in absolute terms (e.g., severity of a symptom, sign, or state of a disease) or as a change from a previous measure. In clinical trials, a PRO instrument can be used to measure the effect of a medical intervention on one or more concepts (i.e., the thing being measured, such as a symptom or group of symptoms, effects on a particular function or group of functions, or a group of symptoms or functions shown to measure the severity of a health condition).