Clinical Trial Management System (CTMS)
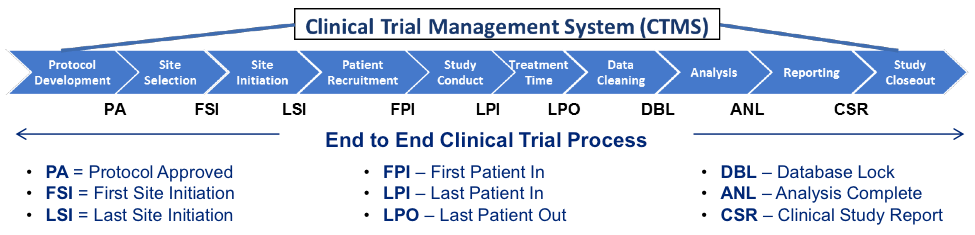
A Clinical Trial Management System (CTMS) is a workflow management application that can support clinical trial management tasks (to manage clinical trials).
- Context:
- It can be focused on different Clinical Trial Phases.
- It can (often) by based on a CTMS Platform (that offers CTMS platform features).
- It can range from being a Physical Site-based CTMS to being a Electronic CTMS.
- It can range from being a Single Site CTMS to being a Multisite CTMS.
- It can range from being a Centralized CTMS to being a DCT CTMS (for DCTs).
- It can be analyzed by a Clinical Trial Management System Capability Analysis.
- It can include a:
- Clinical Trial Audit Trail System,
- Electronic Data Capture (EDC) System,
- Electronic Signature System,
- Clinical Data Management System (CDMS),
- Clinical Data Quality Management System (CQMS),
- Clinical Research Validation System,
- Electronic Clinical Outcome Assessment (eCOA) System.
- Patient Monitoring System (PMS).
- Patient Recruitment System (PRS).
- …
- Example(s):
- Counter-Example(s):
- See: Clinical Research, Decentralized Clinical Trial, Clinical Trial Protocol, Clinical Trial Participant, Clinical Trial Phase, Direct-to-Patient Digital Clinical Trial, Cloud-based Digital Patient Recruitment and Engagement Platform, Siteless Clinical Trial.
References
2022
- HTTP://chat.openai.com
- Q: how is clinical trial management a workflow management task?
- A: Clinical trial management involves the coordination and oversight of all activities related to the conduct of a clinical trial. This includes planning and designing the trial, recruiting and enrolling participants, collecting and analyzing data, and reporting results. Clinical trial management involves a wide range of tasks and activities, many of which can be considered workflow management tasks.
One example of a workflow management task in clinical trial management is the coordination of study visits and assessments for participants. This involves creating a schedule for participants to visit the study site, coordinating with staff to ensure that the necessary assessments and procedures are performed during each visit, and tracking the progress of each participant through the study. Another example is the management of data collection and analysis. This involves creating a plan for collecting and storing data, coordinating with staff to ensure that data is collected and entered correctly, and managing the process of analyzing and interpreting the data.
Overall, clinical trial management requires the coordination and oversight of many different tasks and activities, making it a complex workflow management task.
2021a
- (PSoM, 2021) ⇒ https://www.med.upenn.edu/ocr/about-ctms.html Retrieved:2021-12-19.
- QUOTE: The PennCTMS (Velos) is a clinical trials management system designed to support and manage research in the Perelman School of Medicine (PSoM). This centralized database is designed to help study coordinators, principal investigators, faculty and research staff track and report on nearly every aspect of a clinical trial.
The PennCTMS enables the study team to manage aspects of research such as subject tracking, CRF collection, data management, reporting and financial tools. Study calendars can be created based on the protocol to track milestones. The benefits of this centralized management system include; improved regulatory compliance, visibility into study status and study progress, study data aggregation and financial oversight. The PennCTMS interface with PennChart (EPIC) synchronizes patient enrollment statuses between the two systems, creating one consistent source of enrollment data. Study, patient, and demographic data remain current and accurate in both systems.
- QUOTE: The PennCTMS (Velos) is a clinical trials management system designed to support and manage research in the Perelman School of Medicine (PSoM). This centralized database is designed to help study coordinators, principal investigators, faculty and research staff track and report on nearly every aspect of a clinical trial.
2021b
- (SC CTSI, 2021) ⇒ https://sc-ctsi.org/resources/ctms Retrieved:2021-12-19.
- QUOTE: USC's Clinical Trial Management System, OnCore, is a jointly sponsored, web-based software system for managing clinical trials. The CTMS is integrated with the Electronic Medical Records to streamline the process of managing clinical trials. KeckCare (Keck Medicine of USC) and KIDS (CHLA) Research Administrators, Study Coordinators, Budget Specialists, and Researchers conducting research at USC, LAC and CHLA select the OnCore platform and have worked to customize and configure the CTMS since October 2014 (...)
What is a Clinical Trials Management System (CTMS)?
The CTMS is an online tool for managing all aspects of the clinical-trial lifecycle. This includes study participant and safety management, and electronic data capture and reporting through its core module.
- QUOTE: USC's Clinical Trial Management System, OnCore, is a jointly sponsored, web-based software system for managing clinical trials. The CTMS is integrated with the Electronic Medical Records to streamline the process of managing clinical trials. KeckCare (Keck Medicine of USC) and KIDS (CHLA) Research Administrators, Study Coordinators, Budget Specialists, and Researchers conducting research at USC, LAC and CHLA select the OnCore platform and have worked to customize and configure the CTMS since October 2014 (...)
2021c
- (UW Medicine, 2021) ⇒ https://www.iths.org/ctms/about/what-is-a-clinical-trial-management-system/ Retrieved:2021-12-19.
- QUOTE: ... A clinical trial management system (CTMS) is a software system used to manage clinical trials in clinical research. This CTMS will serve as a single, centralized, web-based enterprise resource to support clinical research studies conducted within or across the three institutions.
2021d
- (Wieczerzak, 2021) ⇒ Michael Wieczerzak (2021)."Electronic Clinical Trial Management Systems: The Basics, Needs, and Outputs". In: SOCRA for Clinical Research Excellence. Retrieved:2021-12-19.
- QUOTE: Day-to-day operations of CTMSs involve many tasks and features including data entry, reporting, and data review. While some systems can communicate with each other, some manual labor may be involved in ensuring that systems are consistent. In some cases, data must be entered manually as it might not be captured in any other location. System syncing with the eCRF, safety database, IxRS, etc. is a major feature of CTMSs. This eliminates human error and makes managing clinical trials much easier.
System oversight is an absolute necessity. Clinical trial teams cannot just rely on the data in CTMS. They must regularly ensure that the data are correct whether they take a risk-based approach or perform 100% audits.
Reporting is another major feature of CTMSs. Management wants to see succinct high-level reports that provide necessary information. Often, these reports are done in an ad hoc manner and are not standardized across clinical trials. Management usually want reports that can easily be used across trials. CTMSs can produce consistent reports that use the same formats and information for different trials and programs.
The Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) emphasizes risk management. CTMS can perform trend analysis such as tracking risks and key quality issues and metrics. This enables sponsors to identify issues and fix them in a timely manner.
Other electronic systems that can be synced with CTMSs include:
- QUOTE: Day-to-day operations of CTMSs involve many tasks and features including data entry, reporting, and data review. While some systems can communicate with each other, some manual labor may be involved in ensuring that systems are consistent. In some cases, data must be entered manually as it might not be captured in any other location. System syncing with the eCRF, safety database, IxRS, etc. is a major feature of CTMSs. This eliminates human error and makes managing clinical trials much easier.
2021e
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Clinical_trial_management_system Retrieved:2021-10-31.
- A Clinical Trial Management System (CTMS) is a software system used by biotechnology and pharmaceutical industries to manage clinical trials in clinical research. The system maintains and manages planning, performing and reporting functions, along with participant contact information, tracking deadlines and milestones.
2021f
- (MassBio, 2021) ⇒ Mansfield, eClinical Solutions (2021) eClinical Solutions Launches elluminate® Clinical Trial Management System (CTMS) for Faster Drug Development. Posted on: 2021-02-01.
- QUOTE: Mansfield, MA, February 1, 2021 — eClinical Solutions LLC, a global provider of cloud-based enterprise software and software-driven clinical data services, today announced the launch of the elluminate® Clinical Trial Management System (CTMS). Built on the elluminate platform, which accelerates digitization and reduces clinical data review cycle times, elluminate CTMS now provides one source of truth for clinical operations data for faster and more informed decision making(...)
For more information on how eClinical Solutions' clients are using elluminate CTMS, please visit: https://www.eclinicalsol.com/products/ctms.
- QUOTE: Mansfield, MA, February 1, 2021 — eClinical Solutions LLC, a global provider of cloud-based enterprise software and software-driven clinical data services, today announced the launch of the elluminate® Clinical Trial Management System (CTMS). Built on the elluminate platform, which accelerates digitization and reduces clinical data review cycle times, elluminate CTMS now provides one source of truth for clinical operations data for faster and more informed decision making(...)
2020
- (Ginige et al., 2020) ⇒ Jeewani Anupama Ginige, Christos Boulamatsis, Megan Heffernan, Juan Carlos San Jose, Igor Chuprov, Tiffany Chau, Anthony Maeder et al. (2020). "Fully-online, Interoperable Clinical Trial Management System for Multi-interventional RCT: Maintain Your Brain Digital Platform". Stud Health Technol Inform, 268.
- QUOTE: Maintain Your Brain (MYB) is a randomised controlled trial (RCT) of multiple online interventions designed to target modifiable risk factors for Alzheimer's disease and dementia. Traditional clinical trial management systems (CTMS) requirements consist of features such as management of the study, site, subject (participant), clinical outcomes, external and internal requests, education, data extraction and reporting, security, and privacy. In addition to fulfilling these traditional requirements, MYB has a specific set of features that needs to be fulfilled. These specific requirements include: (i) support for multiple interventions within a study, (ii) flexible interoperability options with third-party software providers, (iii) study participants being able to engage in online activities via web-based interfaces throughout the trial (from screening to follow-up), (iv) ability to algorithmically personalize trial activities based on the needs of the participant, and (v) the ability to handle large volumes of data over a long period. This paper outlines how the existing CTMSs fall short in meeting these specific requirements. The presented system architecture, development approach and lessons learned in the implementation of the MYB digital platform will inform researchers attempting to implement CTMSs for trials comparable to MYB in the future.
2018
- (Park, Yoon et al., 2018) ⇒ Yu Rang Park, Young Jo Yoon, HaYeong Koo, Soyoung Yoo, Chang-Min Choi, Sung-Ho Beck, and Tae Won Kim. (2018). "Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process As An Integrated Database: System Development". Journal of medical Internet research 20, no. 4.
- QUOTE: The AMC CTMS is an all-in-one system containing all functions needed for operating site-level clinical trials and is a Web-based single sign-on system. As shown in Figure 2, the AMC CTMS is mainly composed of 3-layered domains, namely data integration, management, and utilization. First, the data integration layer of the system includes functions that interface data between the CTMS and legacy systems such as e-IRB, HIS, ERP, and barcode system in the hospital. Data for each existing system are unified and transmitted to the CTMS according to the system-specific interfacing cycle. Depending on the characteristics of the legacy system, the interface methods with the CTMS are different: (1) large scale systems such as the HIS and ERP systems were made through a separate interface server, (2) e-IRB as a Web-based system is linked to a Web service via the Application Programming Interface, and (3) the barcode system as a standalone system is linked through a database to database link. The data management layer of the system includes 7 management applications for basic study management and for managing operational data for clinical trials at the site level. Finally, the data utilization layer of the system is composed of the following 4 applications — report generation, visualization, alert and notification, and task management. The 4 applications in this domain are based on operating data in the CTMS to support clinical trial operations, such as notifications of subject visits to the hospital.
2016
- (NIH, 2016) ⇒ https://www.nidcd.nih.gov/research/clinical-studies/researchers-professionals/understand-clinical-trials-changes-at-NIH
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...