Electronic Clinical Outcome Assessment (eCOA) System
(Redirected from electronic COAs (eCOAs))
Jump to navigation
Jump to search
An Electronic Clinical Outcome Assessment (eCOA) System is an electronic data capture system that can capture and collect clinical outcome assessment measures in electronic format.
- Context:
- It can range from being an ePRO System (for ePROs) to being an eClinRO System (for eClinROs) to being an eObsRO System (for eObsROs) to being a ePerfO System (for ePerfOs).
- …
- Example(s):
- Counter-Example(s):
- a Physical COA System.
- Clinical Endpoint Detection System (CEDS).
- Clinical Trial Management System (CTMS).
- Electronic Clinical Case Report Form (eCRF) System,
- Electronic Informed Consent (eConsent) System,
- Electronic Health Record (eHR) System,
- Electronic Patient Diary (eDiary) System,
- Paper-based Clinical Outcome Assessment (COA) System,
- Patient Monitoring System (PMS),
- Patient Recruitment System (PRS),
- Patient Retention System.
- See: Decentralized Clinical Trial, Bring Your Own Device (BYOD) Clinical Trial, Direct-to-Patient Digital Clinical Trial, Patient eDiary, Software as a Medical Device (SaMD), Digital Biomarker Collection System, Cloud-based Digital Patient Recruitment and Engagement Platform, Interactive Voice Response (IVR) System.
References
2021a
- (DIA, 2021) ⇒ https://engage.diaglobal.org/Whitepapers-CRFHealth.html Retrieved:2021-12-19.
- QUOTE: A clinical outcome assessment (COA) measures a patient’s symptoms, as well as his or her mental state, or the effects of a disease or condition on a patient. Some are unobservable concepts, such as pain intensity, moods or feelings, and eating habits. Electronic clinical outcome assessments, or eCOAs, employs technology such as smartphones, tablets, and personal computers to allow patients, clinicians, and their caregivers to directly report outcomes.
2021b
- (Signant Health, 2021) ⇒ https://www.signanthealth.com/solutions/clinical-outcome-assessments/smartsignals-ecoa-ecoa/ Retrieved:2021-12-12.
- QUOTE: The difference between Electronic Clinical Outcome Assessment (eCOA) and Electronic Patient-Reported Outcome (ePRO) is that ePRO systems are a type of eCOA. Clinical trial patients often use eCOA system technologies on mobile devices to remotely send their ePRO symptoms and experience directly to site staff. Other types of COAs include Electronic Performance Outcome (ePerfO), Electronic Clinician-Reported Outcome (eClinRO), and Electronic Observer-Reported Outcome (eObsRO).
2021c
- (FDA, 2021) ⇒ https://www.fda.gov/about-fda/clinical-outcome-assessment-coa-frequently-asked-questions#COADefinition
- QUOTE: A clinical outcome assessment is a measure that describes or reflects how a patient feels, functions, or survives. Types of COAs include:
2021d
- (Physio-pedia, 2021) ⇒ https://www.physio-pedia.com/Clinical_Outcome_Assessment
- QUOTE:
- An outcome assessment, the patient assessment used in an endpoint, is the measuring instrument that provides a rating or score (categorical or continuous) that is intended to represent some aspect of the patient’s health status.
- Outcome assessments are used to define efficacy endpoints when developing a therapy for a disease or condition.
- Most efficacy endpoints are based on specified clinical assessments of patients.
- When clinical assessments are used as clinical trial outcomes, they are called clinical outcome assessments (COAs).
- QUOTE:
2020
- (Medable, 2020) ⇒ https://blog.medable.com/author/dr-ingrid-oakley-girvan
- QUOTE: ... In patient-centric clinical trials, COAs are essential to understand the impacts of a drug on certain endpoints, for example, whether it is improving or diminishing quality of life and everyday activities that matter to patients. The FDA, signaling the importance of COAs and the need to develop additional ones, has even created specific guidance and pathways for their development and validation. ...
...
- QUOTE: ... In patient-centric clinical trials, COAs are essential to understand the impacts of a drug on certain endpoints, for example, whether it is improving or diminishing quality of life and everyday activities that matter to patients. The FDA, signaling the importance of COAs and the need to develop additional ones, has even created specific guidance and pathways for their development and validation. ...
2019
- (NHI, 2019) ⇒ https://nationalhealthcouncil.org/blog/blog-fdas-roadmap-patient-focused-outcome-measurement-clinical-trials/
- QUOTE: ... Patient groups can help advance patient-centered drug development by the having information needed in column one at the ready. That can save considerable time and effort to speed the process and ensure the patient voice is accurately captured. ...
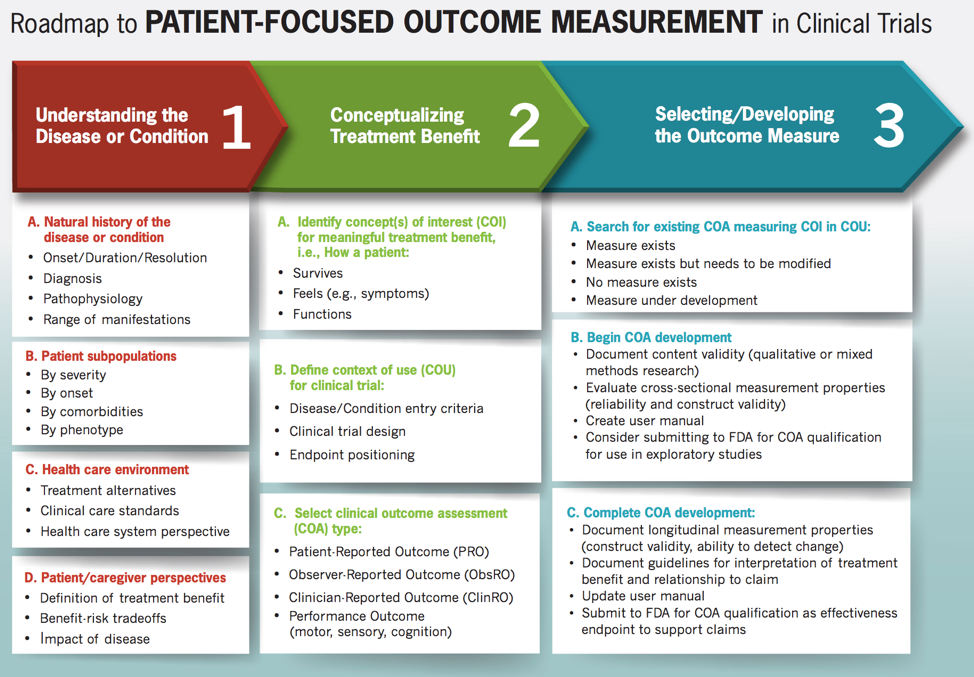
- QUOTE: ... Patient groups can help advance patient-centered drug development by the having information needed in column one at the ready. That can save considerable time and effort to speed the process and ensure the patient voice is accurately captured. ...
2017
- (Feaster et al., 2017) ⇒ H. Todd Feaster, Todd M. Solomon, Danielle Abi-Saab, Annamarie Vogt, Jordan M. Barbone, John Harrison, and David S. Miller (2017)"[P3–015: The Impact Of Electronic Clinical Outcome Assessments (Ecoa) On Alzheimer'S Disease Clinical Trial Data Quality"]. In: Alzheimer's Association International Conference: P3: Poster Presentations. DOI:10.1016/j.jalz.2017.06.1828.
- QUOTE: The Alzheimer's Disease Assessment Scale – Cognitive subscale (ADAS-Cog) and Mini-Mental State Examination (MMSE) are two of the most commonly used scales in Alzheimer's disease (AD) clinical trials. Previous research has demonstrated error rates among clinical trial raters to be upwards of 32% for the ADAS-Cog and 23% for the MMSE on initial administration when using a paper-pencil version of the scale. The original electronic Clinical Outcome Assessments (eCOA) platform reduced ADAS-Cog and MMSE error rates in the WN28745 Marguerite RoAD trial to 11.3% and 10.8% respectively. The platform and scales were recently enhanced and improved and are currently utilized in the BN29552 CREAD Study. We evaluated their impact on overall data quality in comparison to the initial versions of the scales.
2015
- (Tolley et al., 2015) ⇒ Chloe Tolley, Diana Rofail, Adam Gater, and Justine K Lalonde (2015). "The feasibility of using electronic clinical outcome assessments in people with schizophrenia and their informal caregivers". In: Patient Related Outcome Measures 6(9). DOI:10.2147/PROM.S79348.
- QUOTE: Traditionally, COAs have been developed and validated using pen and paper. However, as digital technology has evolved, the use of electronic COAs (eCOAs) has become more prevalent. The use of eCOAs is associated with a number of advantages over pen-and-paper methods, particularly in terms of reducing missing data, providing time-stamped records, minimizing administrative burden, and avoiding secondary data entry errors[1]. When migrating an instrument from a paper to an electronic mode of administration, the resulting eCOA ought to produce data that are equivalent or superior to the data produced from the original paper version and this equivalency should be solely due to the change in mode . In addition, it is essential to demonstrate the “usability” of the eCOA (ie, that respondents are able to use the software and the device appropriately) .
- ↑ Coons SJ, Gwaltney CJ, Hays RD, et al. ISPOR ePRO Task Force Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health. 2009;12:419–429.