Clinical Trial Management Process
(Redirected from Clinical Trials Management)
Jump to navigation
Jump to search
A Clinical Trial Management Process is a clinical study process that manages clinical trials.
- Context:
- Input: Clinical Trial Specification.
- It can (typically) include Project Management Task.
- It can (typically) include Clinical Trial Management Sub-Tasks.
- It can (often) be supported by a Clinical Trial Management System (CTMS).
- …
- Example(s):
- Clinical Trial Audit Trail Task, for NCT00437424 Brivanib Clinical Trial.
- Electronic Data Capture Task, for NCT00437424 Brivanib Clinical Trial.
- Electronic Signature Task, for NCT00437424 Brivanib Clinical Trial.
- Clinical Data Management Task, for NCT00437424 Brivanib Clinical Trial.
- Clinical Data Quality Management Task, for NCT00437424 Brivanib Clinical Trial.
- Clinical Research Validation Task, for NCT00437424 Brivanib Clinical Trial.
- Clinical Outcome Assessment Task.
- Clinical Trial Patient Monitoring Task / Patient Monitoring Task.
- Clinical Trial Patient Recruitment Task.
- Clinical Trial Medical Coding Task.
- Clinical Trial Data Management Task.
- …
- Counter-Example(s):
- See: Clinical Trial Process, Clinical Research, Decentralized Clinical Trial, Clinical Trial Protocol, Clinical Trial Participant, Clinical Trial Phase.
References
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Clinical_trial_management_system Retrieved:2021-10-31.
- A Clinical Trial Management System (CTMS) is a software system used by biotechnology and pharmaceutical industries to manage clinical trials in clinical research. The system maintains and manages planning, performing and reporting functions, along with participant contact information, tracking deadlines and milestones.
2016
- (NIH, 2016) ⇒ https://www.nidcd.nih.gov/research/clinical-studies/researchers-professionals/understand-clinical-trials-changes-at-NIH
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...
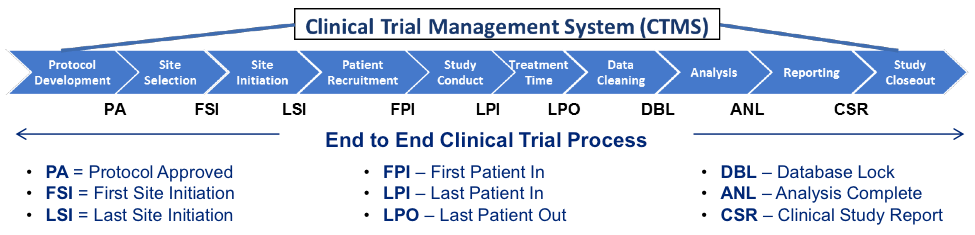
- QUOTE: For several years, increasing numbers of life sciences organizations have implemented a Clinical Trial Management System (CTMS) that can provide insights gleaned from the system’s data to gain early and increased visibility into problems, progress and possibilities. Many organizations have a constant need to expand CTMS capabilities, integrate clinical operations data across multiple systems, and update clinical trial processes – all in order to adapt to changing regulatory requirements and clinical trial practices. ...
2015
- (Gupta et al., 2015) ⇒ Anjali Gupta, Karen J. Calfas, Simon J. Marshall, Thomas N. Robinson, Cheryl L. Rock, Jeannie S. Huang, Melanie Epstein-Corbin et al. (2015). “Clinical Trial Management of Participant Recruitment, Enrollment, Engagement, and Retention in the SMART Study Using a Marketing and Information Technology (MARKIT) Model.” Contemporary Clinical Trials, 42
- ABSTRACT: Advances in information technology and near ubiquity of the Internet have spawned novel modes of communication and unprecedented insights into human behavior via the digital footprint. Health behavior randomized controlled trials (RCTs), especially technology-based, can leverage these advances to improve the overall clinical trials management process and benefit from improvements at every stage, from recruitment and enrollment to engagement and retention. In this paper, we report the results for recruitment and retention of participants in the SMART study and introduce a new model for clinical trials management that is a result of interdisciplinary team science. The MARKIT model brings together best practices from information technology, marketing, and clinical research into a single framework to maximize efforts for recruitment, enrollment, engagement, and retention of participants into a RCT. These practices may have contributed to the study's on-time recruitment that was within budget, 86% retention at 24 months, and a minimum of 57% engagement with the intervention over the 2-year RCT. Use of technology in combination with marketing practices may enable investigators to reach a larger and more diverse community of participants to take part in technology-based clinical trials, help maximize limited resources, and lead to more cost-effective and efficient clinical trial management of study participants as modes of communication evolve among the target population of participants.