Clinical Data Interchange Standards Consortium (CDISC) Foundational Standard
Jump to navigation
Jump to search
A Clinical Data Interchange Standards Consortium (CDISC) Foundational Standard is a CDISC Standard that is focused on defining standards for preclinical/clinical trial data and includes models, domains and specifications for data representation.
- Context:
- It (typically) consists of a complete set of standards for supporting clinical and non-clinical research processes from end-to-end.
- It (usually) doesn't include controlled-terminology, semantics or data exchange standards.
- …
- Example(s):
- Counter-Example(s):
- See: Clinical Data Interchange Standards Consortium (CDISC) RWD Connect Initiative, Standard-Developing Organization, Clinical Trial Data, Clinical Data Standards, CDISC Shared Health And Research Electronic library (SHARE), CDISC Operational Data Model (ODM), CDISC BRIDG Model. CDISC SHARE Application Programming Interface (API), CDISC SHARE Software Ecosystem.
References
2022a
- (CDISC, 2022) ⇒ https://www.cdisc.org/standards/foundational Retrieved:2022-2-25.
- QUOTE: CDISC Foundational Standards are the basis of a complete suite of data standards, enhancing the quality, efficiency and cost effectiveness of clinical research processes from beginning to end. Foundational Standards focus on the core principles for defining data standards and include models, domains and specifications for data representation.
- Protocol Representation Model (PRM) provides a standard for planning and designing a research protocol with focus on study characteristics such as study design, eligibility criteria, and requirements from the ClinicalTrials.gov, World Health Organization (WHO) registries, and EudraCT registries. PRM assists in automating CRF creation and EHR configuration to support clinical research and data sharing.
- Standard for Exchange of Nonclinical Data (SEND)[1] is an implementation of the SDTM standard for nonclinical studies. SEND specifies a way to collect and present nonclinical data in a consistent format.
- Clinical Data Acquisition Standards Harmonization (CDASH) establishes a standard way to collect data in a similar way across studies and sponsors so that data collection formats and structures provide clear traceability of submission data into the Study Data Tabulation Model (SDTM), delivering more transparency to regulators and others who conduct data review.
- Study Data Tabulation Model (SDTM)[2] provides a standard for organizing and formatting data to streamline processes in collection, management, analysis and reporting.
- Study Data Tabulation Model Implementation Guide (SDTMIG)[2] is intended to guide the organization, structure, and format of standard clinical trial tabulation datasets.
- Analysis Data Model (ADaM)[2] defines dataset and metadata standards that support:
- (...)
- ↑ Required by the FDA (U.S.) for new drug applications
- ↑ 2.0 2.1 2.2 Required by the FDA and PMDA (Japan) for new drug applications.
2022b
- (Facile et al., 2022) ⇒ Rhonda Facile, Erin Elizabeth Muhlbradt, Mengchun Gong, Qingna Li, Vaishali Popat, Frank Petavy, Ronald Cornet, Yaoping Ruan, Daisuke Koide, Toshiki I. Saito, Sam Hume, Frank Rockhold, Wenjun Bao, Sue Dubman, Barbara Jauregui Wurst (2022). "Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey". In: JMIR medical informatics, 10(1), e30363.
- QUOTE: The CDISC standards span the clinical research process and include standards for the exchange of nonclinical data (SEND), data collection case report forms (CRFs; clinical data acquisition standards harmonization (CDASH)), aggregation and tabulation (study data tabulation model (SDTM)), Biomedical Research Integrated Domain Group (BRIDG) logical model, and operational data model (ODM) for transport (Figure 1). In collaboration with the National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) program, CDISC has developed a rich controlled terminology that is linked to other common research semantics through the NCI-EVS tools. These standards, presented in data models, implementation guides, and user guides, are globally recognized and heavily used by the biopharmaceutical industry and some academic institutions.
2022c
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/Clinical_Data_Interchange_Standards_Consortium#Overview_of_standards Retrieved:2022-2-25.
- Dataset.XML (DataSet-XML)
- Enables communication of study results as well as regulatory submission to FDA (pilot since 2014).
- Study Data Tabulation Model (SDTM)
- Recommended for FDA regulatory submissions since 2004.
- The SDTM Implementation Guide (SDTM-IG) gives a standardized, predefined collection of domains for clinical data submission, each of which is based on the structure and metadata defined by the SDTM.
- Standard for Exchange of Non-clinical Data (SEND).
- The SEND Implementation Guide (SEND-IG) provides predefined domains and examples of non-clinical (animal) data based on the structure and metadata defined by the SDTM.
- Analysis Data Model (ADaM)
- Defines dataset and metadata standards that support statistical analyses and traceability. ADaM is one of the required standards for data submission to FDA (U.S.) and PMDA (Japan).
- Operational Data Model (ODM).
- The highlights of ODM: includes audit trail, utilizes XML technology, machine- and human- readable, all information are independent from databases, storing of ODM is independent from hard- and software.
- Laboratory Data Model (LAB)
- The Lab standard is used for exchange of laboratory data between labs and CROs
- Case Report Tabulation Data Definition Specification (CRT-DDS)
- Also referred to as “define.xml", a machine readable version of the regulatory submission "define.pdf".
- Clinical Data Acquisition Standards Harmonization (CDASH)
- Defines a minimal data collection set for sixteen safety SDTM Domains, harmonizing element names, definitions and metadata. The objective is to establish a standardized data collection baseline across all submissions.
- CDISC Terminology
- Defines controlled terminology for SDTM and CDASH, provides extensible lists of controlled terms designed to harmonize data collected across submissions.
- Dataset.XML (DataSet-XML)
2018
- (Hume et al., 2018) ⇒ Samuel Hume, Anthony Chow, Julie Evans, Frederik Malfait, Julie Chason, J. Darcy Wold, Wayne Kubick,and Lauren B. Becnel (2018). "CDISC SHARE, a Global, Cloud-based Resource of Machine-Readable CDISC Standards for Clinical and Translational Research". In: AMIA Summits on Translational Science Proceedings, 2018, 94.
- QUOTE: CDISC standards (Figure 1) are categorized as Foundational Standards, Semantics, Therapeutic Area (TA) Standards, and Transport Standards. Foundational Standards include Standard for Exchange of Nonclinical Data (SEND)[1] for the collection and tabulation of animal model and other pre-clinical data, Protocol Representation Model (PRM)[2], Analysis Data Model (ADaM)[3] for defining analysis datasets, Clinical Data Acquisition Standards Harmonization (CDASH)[4] that provide a minimal set of data elements common to essentially all studies, the Study Data Tabulation Model (SDTM)[5] for data tabulation, and others.

|
- Except for Protocol, the foundational standards and their implementation guides are organized into classes of related categories of information. Within SDTM for example, each class is subdivided into domains, and each domain is comprised of variables. Semantics include CDISC Controlled Terminologies, developed in collaboration with NCI EVS, and the Biomedical Research Integrated Domains Group (BRIDG) domain information model. TA User Guides provide indication-specific “slices” of foundational standards and their terminologies, with examples of how to use these standards for a given TA. Transport Standards support submissions, data interchange, study archival, and automated study setup[6].
- ↑ Standard for Exchange of Nonclinical Data (SEND) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/send.
- ↑ CDISC. CDISC Protocol Representation Model Version 1.0. http://cdisc.org/ CDISC. 2010.
- ↑ Analysis Data Model (ADaM) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/adam
- ↑ Clinical Data Acquisition Standards Harmonization (CDASH) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/cdash.
- ↑ Study Data Tabulation Model (SDTM) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/sdtm.
- ↑ Hume S, Aerts J, Sarnikar S, Huser V. Current applications and future directions for the CDISC Operational Data Model standard: A methodological review. Journal of Biomedical Informatics. 2016
2017
- (AMED, 2017) ⇒ https://www.amed.go.jp/en/aboutus/collaboration/cdisc.html Last updated 2017-4-7.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) develops and supports global and platform-independent data standards that enable information system interoperability to improve medical research and related areas of healthcare. It is an international interdisciplinary NPO established in 1997. In October 2016, the PMDA began accepting submissions of electronic clinical trial data conforming to CDISC standards by applicants of manufacture and marketing authorization of new drugs (this procedure was implemented by the FDA in previously years). In the future, conducting clinical trials including investigator initiated ones complied with to the CDISC standards will become necessary from the planning and implementation stages (...)
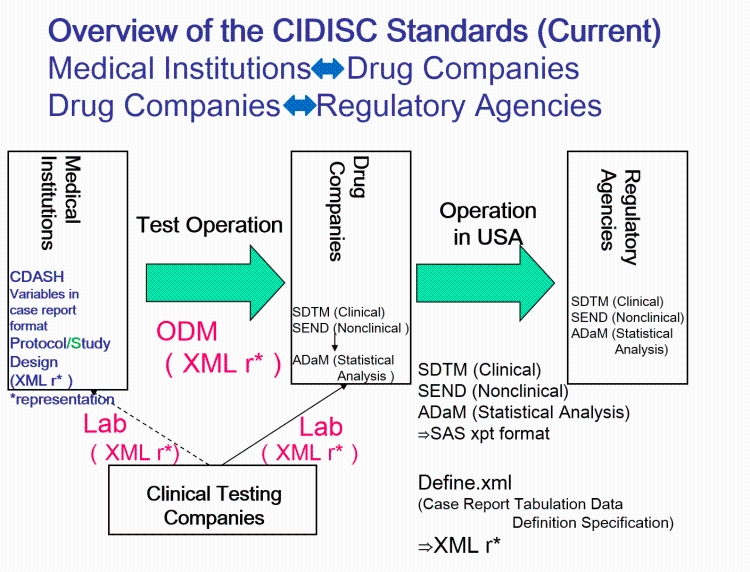
2016
- (Hume et al., 2016) ⇒ Sam Hume, Jozef Aerts, Surendra Sarnikar, and Vojtech Huserc (2016)."Current Applications and Future Directions for the CDISC Operational Data Model Standard: A Methodological Review". In: Journal of biomedical informatics, 60:352-362. DOI:10.1016/j.jbi.2016.02.016
- QUOTE: Figure 1 highlights the CDISC foundational standards covered by ODM, and standardized extensions such as Clinical Data Acquisition Standards Harmonization (CDASH) that describes the basic data collection fields for domains, the Study Data Tabulation Model (SDTM) that describes a standard structure for study data tabulations, and the Analysis Data Model (ADaM) that describes metadata models and examples for analysis datasets (...)

|
