Clinical Data Interchange Standards Consortium (CDISC)
A Clinical Data Interchange Standards Consortium (CDISC) is a standard-developing organization focused on medical research data linked with healthcare.
- Context:
- Website: https://www.cdisc.org
- It can produce a CDISC Standard, such as CDISK Base and CDISC Plus.
- It can be organized by the following standards:
- It includes a CDISC Shared Health And Research Electronic library (SHARE), CDISC Operational Data Model (ODM), CDISC BRIDG Model.
- Example(s):
- Counter-Example(s):
- See: European Committee For Standardization, Standard-Developing Organization, HL7, International Organization For Standardization, CDISC Library, Clinical Trial Data, Clinical Data Standards, National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) Program, CDISC SHARE Application Programming Interface (API), CDISC SHARE Software Ecosystem.
References
2022
- (Facile et al., 2022) ⇒ Rhonda Facile, Erin Elizabeth Muhlbradt, Mengchun Gong, Qingna Li, Vaishali Popat, Frank Petavy, Ronald Cornet, Yaoping Ruan, Daisuke Koide, Toshiki I. Saito, Sam Hume, Frank Rockhold, Wenjun Bao, Sue Dubman, Barbara Jauregui Wurst (2022). "Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey". In: JMIR medical informatics, 10(1), e30363.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) RWD Connect Initiative was designed to better understand the barriers to implementing CDISC standards for RWD and to obtain a picture of what tools and guidance may be needed to implement CDISC standards more easily for this purpose.
In the world of traditional clinical trials, which are undertaken with the intent of submitting a new medical product or intervention to regulatory authorities such as the US Food and Drug Administration (FDA) or the Japanese Pharmaceutical and Medical Devices Agency for marketing authorization approval, a set of global data standards has been adopted and is being required by an increasing number of national and regional regulatory agencies. These standards were developed through CDISC, a global nonprofit organization that started >20 years ago to generate open-access platform-agnostic data standards for clinical research and its link to health care.
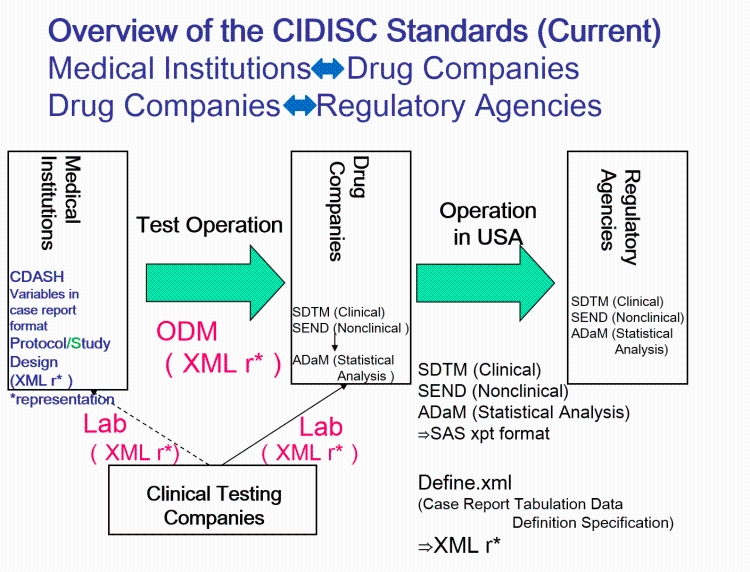
The CDISC standards span the clinical research process and include standards for the exchange of nonclinical data (SEND), data collection case report forms (CRFs; clinical data acquisition standards harmonization (CDASH)), aggregation and tabulation (study data tabulation model (SDTM)), Biomedical Research Integrated Domain Group (BRIDG) logical model, and operational data model (ODM) for transport (Figure 1). In collaboration with the National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) program, CDISC has developed a rich controlled terminology that is linked to other common research semantics through the NCI-EVS tools. These standards, presented in data models, implementation guides, and user guides, are globally recognized and heavily used by the biopharmaceutical industry and some academic institutions.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) RWD Connect Initiative was designed to better understand the barriers to implementing CDISC standards for RWD and to obtain a picture of what tools and guidance may be needed to implement CDISC standards more easily for this purpose.
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Clinical_Data_Interchange_Standards_Consortium Retrieved:2021-10-12.
- The Clinical Data Interchange Standards Consortium (CDISC) is a standards developing organization (SDO) dealing with medical research data linked with healthcare, to "enable information system interoperability to improve medical research and related areas of healthcare". The standards support medical research from protocol through analysis and reporting of results and have been shown to decrease resources needed by 60% overall and 70–90% in the start-up stages when they are implemented at the beginning of the research process.
CDISC standards are harmonized through a model that is also a HL7 standard and is the process to becoming an ISO/CEN standard.
- The Clinical Data Interchange Standards Consortium (CDISC) is a standards developing organization (SDO) dealing with medical research data linked with healthcare, to "enable information system interoperability to improve medical research and related areas of healthcare". The standards support medical research from protocol through analysis and reporting of results and have been shown to decrease resources needed by 60% overall and 70–90% in the start-up stages when they are implemented at the beginning of the research process.
2021
- (CDISC, 2021) ⇒ https://www.cdisc.org/cdisc-library
- QUOTE: Listed below are the available CDISC standards and terminology. This page will be periodically updated to reflect new released content.
- CDASH/CDASHIG.
- CDASH Model v1.0, v1.1, v1.2
- CDASHIG v2.0, v2.1, v2.2
- CDASH v1.1/CDASHUG v1.0
- SDTM.
- SDTM v1.2, v1.3, v1.4, v1.5, v1.6, v1.7, v1.8, v2.0
- SDTMIG.
- SDTMIG v3.1.2, v3.1.3, v3.2, v3.3, v3.4
- SDTMIG-AP v1.0
- SDTMIG-MD v1.0, v1.1
- SDTMIG-PGx v1.0
- SENDIG.
- SENDIG v3.0, v3.1, 3.1.1
- SENDIG-DART v1.1
- SENDIG-AR v1.0
- ADaMIG.
- ADaMIG v1.0, v1.1, v1.2, v1.3
- ADaM ADAE v1.0
- ADaM BDS for TTE v1.0
- ADaM OCCDS v1.0, v1.1
- ADaMIG NCA v1.0
- ADaMIG MD v1.0
- QRS.
- Functional Test
- SIX MINUTE WALK v1.0
- Questionnaire
- CGI v2.1
- PGI v1.1
- Functional Test
- Controlled Terminology.
- P19 (2014-09-26) to P48 (2021-12-17): 30 quarters
- CDASH/CDASHIG.
- QUOTE: Listed below are the available CDISC standards and terminology. This page will be periodically updated to reflect new released content.
2018
- (Hume et al., 2018) ⇒ Samuel Hume, Anthony Chow, Julie Evans, Frederik Malfait, Julie Chason, J. Darcy Wold, Wayne Kubick,and Lauren B. Becnel (2018). "CDISC SHARE, a Global, Cloud-based Resource of Machine-Readable CDISC Standards for Clinical and Translational Research". In: AMIA Summits on Translational Science Proceedings, 2018, 94.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) is a global non-profit standards development organization that creates consensus-based standards for clinical and translational research. Several of these standards are now required by regulators for electronic submissions of regulated clinical trials' data and by government funding agencies. These standards are free and open, available for download on the CDISC Website as PDFs. While these documents are human readable, they are not amenable to ready use by electronic systems. CDISC launched the CDISC Shared Health And Research Electronic library (SHARE) to provide the standards metadata in machine-readable formats to facilitate the automated management and implementation of the standards (...)
CDISC SHARE Software Ecosystem. The CDISC SHARE Ecosystem is a software tools platform that complements the CDISC SHARE MDR by extending additional services to standards developers and implementers. The CDISC SHARE Ecosystem includes CDISC's instance of Atlassian Confluence Wiki for active standards development and publication support; JIRA for issue tracking and community public review; Bitbucket for collaborative development and version control; and numerous bespoke software applications for loading and extracting MDR content (...)
CDISC Standards Organization. CDISC standards (Figure 1) are categorized as Foundational Standards, Semantics, Therapeutic Area (TA) Standards, and Transport Standards(...)
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) is a global non-profit standards development organization that creates consensus-based standards for clinical and translational research. Several of these standards are now required by regulators for electronic submissions of regulated clinical trials' data and by government funding agencies. These standards are free and open, available for download on the CDISC Website as PDFs. While these documents are human readable, they are not amenable to ready use by electronic systems. CDISC launched the CDISC Shared Health And Research Electronic library (SHARE) to provide the standards metadata in machine-readable formats to facilitate the automated management and implementation of the standards (...)

|
2017
- (AMED, 2017) ⇒ https://www.amed.go.jp/en/aboutus/collaboration/cdisc.html Last updated 2017-4-7.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) develops and supports global and platform-independent data standards that enable information system interoperability to improve medical research and related areas of healthcare. It is an international interdisciplinary NPO established in 1997. In October 2016, the PMDA began accepting submissions of electronic clinical trial data conforming to CDISC standards by applicants of manufacture and marketing authorization of new drugs (this procedure was implemented by the FDA in previously years). In the future, conducting clinical trials including investigator initiated ones complied with to the CDISC standards will become necessary from the planning and implementation stages (...)
2016
- (Cohen Veterans Bioscience, 2016) ⇒ https://www.cohenveteransbioscience.org/2016/06/23/clinical-data-interchange-standards-consortium-cdisc/ Posted on June 23, 2016.
- QUOTE: CDISC is a global, open, multidisciplinary, non-profit organization that has established standards to support the acquisition, exchange, submission and archive of clinical research data and metadata. The CDISC mission is to develop and support global, platform-independent data standards that enable information system interoperability to improve medical research and related areas of healthcare. CDISC standards are vendor-neutral, platform-independent and freely available via the CDISC website.
The CDISC Model

2015
- (Huser et al., 2015) ⇒ Vojtech Huser, Chandan Sastry, Matthew Breymaier, Asma Idriss, and James J.Cimino (2015). "Standardizing data exchange for clinical research protocols and case report forms: An assessment of the suitability of the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM)". In: Journal of Biomedical Informatics, 57:88-99.
- QUOTE: The most relevant standard for representing clinical study data, applicable to unregulated as well as regulated studies, is the Operational Data Model (ODM) in development since 1999 by the Clinical Data Interchange Standards Consortium (CDISC). ODM’s initial objective was exchange of case report forms data but it is increasingly utilized in other contexts. An ODM extension called Study Design Model, introduced in 2011, provides additional protocol representation elements.

