Insulin Molecule
Jump to navigation
Jump to search
↑ Cite error: Invalid
↑ Cite error: Invalid
↑ Drug Information Portal NLM – Insulin human USAN druginfo.nlm.nih.gov
↑ Cite error: Invalid
An Insulin Molecule is a peptide hormone composed of two peptide chains (A chain and B chain) linked together by two disulfide bonds, and an additional disulfide is formed within the A chain.
- Context:
- It can range from being a Organism-Created Insulin (e.g. Human insulin) to being a Manufactured Insulin.
- It can be an Anabolic Hormone (promoting the conversion of small molecules in the blood into large molecules inside the cells).
- It can be a Diabetes Drug (for type 1 diabetes and type 2 diabetes.
- …
- Example(s):
- In most species, the A chain consists of 21 amino acids and the B chain of 30 amino acids.
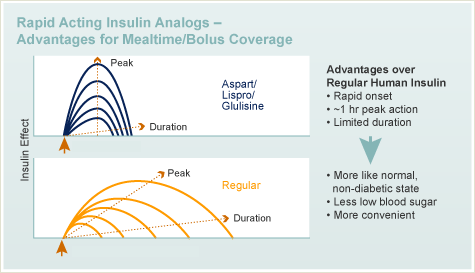
- Rapid-Acting Insulin Analog.
- Long-Acting Insulin.
- Premixed Insulin.
- Short-Acting Insulin.
- …
- Counter-Example(s):
- See: Peptide Hormone, Beta Cell, Pancreatic Islets.
References
2022
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/insulin Retrieved:2022-6-5.
- Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the INS gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of glucose from the blood into liver, fat and skeletal muscle cells. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially of reserve body fat. Beta cells are sensitive to blood sugar levels so that they secrete insulin into the blood in response to high level of glucose, and inhibit secretion of insulin when glucose levels are low. Insulin enhances glucose uptake and metabolism in the cells, thereby reducing blood sugar level. Their neighboring alpha cells, by taking their cues from the beta cells, secrete glucagon into the blood in the opposite manner: increased secretion when blood glucose is low, and decreased secretion when glucose concentrations are high. Glucagon increases blood glucose level by stimulating glycogenolysis and gluconeogenesis in the liver. The secretion of insulin and glucagon into the blood in response to the blood glucose concentration is the primary mechanism of glucose homeostasis. Decreased or absent insulin activity results in diabetes mellitus, a condition of high blood sugar level (hyperglycaemia). There are two types of the disease. In diabetes mellitus type 1, the beta cells are destroyed by an autoimmune reaction so that insulin can no longer be synthesized or be secreted into the blood.[1] In diabetes mellitus type 2, the destruction of beta cells is less pronounced than in type 1, and is not due to an autoimmune process. Instead, there is an accumulation of amyloid in the pancreatic islets, which likely disrupts their anatomy and physiology. The pathogenesis of type 2 diabetes is not well understood but reduced population of islet beta-cells, reduced secretory function of islet beta-cells that survive, and peripheral tissue insulin resistance are known to be involved.[2] Type 2 diabetes is characterized by increased glucagon secretion which is unaffected by, and unresponsive to the concentration of blood glucose. But insulin is still secreted into the blood in response to the blood glucose. As a result, glucose accumulates in the blood. The human insulin protein is composed of 51 amino acids, and has a molecular mass of 5808 Da. It is a heterodimer of an A-chain and a B-chain, which are linked together by disulfide bonds. Insulin's structure varies slightly between species of animals. Insulin from animal sources differs somewhat in effectiveness (in carbohydrate metabolism effects) from human insulin because of these variations. Porcine insulin is especially close to the human version, and was widely used to treat type 1 diabetics before human insulin could be produced in large quantities by recombinant DNA technologies.[3] Insulin was the first peptide hormone discovered. Frederick Banting and Charles Herbert Best, working in the laboratory of J. J. R. Macleod at the University of Toronto, were the first to isolate insulin from dog pancreas in 1921. Frederick Sanger sequenced the amino acid structure in 1951, which made insulin the first protein to be fully sequenced.[4] The crystal structure of insulin in the solid state was determined by Dorothy Hodgkin in 1969. Insulin is also the first protein to be chemically synthesised and produced by DNA recombinant technology. It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.
- Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the INS gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of glucose from the blood into liver, fat and skeletal muscle cells. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially of reserve body fat. Beta cells are sensitive to blood sugar levels so that they secrete insulin into the blood in response to high level of glucose, and inhibit secretion of insulin when glucose levels are low. Insulin enhances glucose uptake and metabolism in the cells, thereby reducing blood sugar level. Their neighboring alpha cells, by taking their cues from the beta cells, secrete glucagon into the blood in the opposite manner: increased secretion when blood glucose is low, and decreased secretion when glucose concentrations are high. Glucagon increases blood glucose level by stimulating glycogenolysis and gluconeogenesis in the liver. The secretion of insulin and glucagon into the blood in response to the blood glucose concentration is the primary mechanism of glucose homeostasis. Decreased or absent insulin activity results in diabetes mellitus, a condition of high blood sugar level (hyperglycaemia). There are two types of the disease. In diabetes mellitus type 1, the beta cells are destroyed by an autoimmune reaction so that insulin can no longer be synthesized or be secreted into the blood.[1] In diabetes mellitus type 2, the destruction of beta cells is less pronounced than in type 1, and is not due to an autoimmune process. Instead, there is an accumulation of amyloid in the pancreatic islets, which likely disrupts their anatomy and physiology. The pathogenesis of type 2 diabetes is not well understood but reduced population of islet beta-cells, reduced secretory function of islet beta-cells that survive, and peripheral tissue insulin resistance are known to be involved.[2] Type 2 diabetes is characterized by increased glucagon secretion which is unaffected by, and unresponsive to the concentration of blood glucose. But insulin is still secreted into the blood in response to the blood glucose. As a result, glucose accumulates in the blood. The human insulin protein is composed of 51 amino acids, and has a molecular mass of 5808 Da. It is a heterodimer of an A-chain and a B-chain, which are linked together by disulfide bonds. Insulin's structure varies slightly between species of animals. Insulin from animal sources differs somewhat in effectiveness (in carbohydrate metabolism effects) from human insulin because of these variations. Porcine insulin is especially close to the human version, and was widely used to treat type 1 diabetics before human insulin could be produced in large quantities by recombinant DNA technologies.[3] Insulin was the first peptide hormone discovered. Frederick Banting and Charles Herbert Best, working in the laboratory of J. J. R. Macleod at the University of Toronto, were the first to isolate insulin from dog pancreas in 1921. Frederick Sanger sequenced the amino acid structure in 1951, which made insulin the first protein to be fully sequenced.[4] The crystal structure of insulin in the solid state was determined by Dorothy Hodgkin in 1969. Insulin is also the first protein to be chemically synthesised and produced by DNA recombinant technology. It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.
<ref> tag; no text was provided for refs named urlInsulin Injection - PubMed Health<ref> tag; no text was provided for refs named Biochemistry<ref> tag; no text was provided for refs named Stretton_20022022
- https://www.vox.com/2019/4/3/18293950/why-is-insulin-so-expensive
- QUOTE: ... According to a 2017 Lancet paper on insulin price increases, “Older insulins have been successively replaced with newer, incrementally improved products covered by numerous additional patents.” The result is that more than 90 percent of privately insured patients with Type 2 diabetes in America are prescribed the latest and costliest versions of insulin. ... One real solution to the problem, however, would be to bring a generic version of insulin to the market. There are currently no true generic options available (though there are several rebranded and biosimilar insulins). This is in part because companies have made those incremental improvements to insulin products, which has allowed them to keep their formulations under patent, and because older insulin formulations have fallen out of fashion. ...
:format(webp):no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/8500453/ioi150073f1.png)
- QUOTE: ... According to a 2017 Lancet paper on insulin price increases, “Older insulins have been successively replaced with newer, incrementally improved products covered by numerous additional patents.” The result is that more than 90 percent of privately insured patients with Type 2 diabetes in America are prescribed the latest and costliest versions of insulin. ... One real solution to the problem, however, would be to bring a generic version of insulin to the market. There are currently no true generic options available (though there are several rebranded and biosimilar insulins). This is in part because companies have made those incremental improvements to insulin products, which has allowed them to keep their formulations under patent, and because older insulin formulations have fallen out of fashion. ...
2021
1923
- Insulin Patent