Clinical Data Interchange Standards Consortium (CDISC) Standard
(Redirected from CDISC Standards)
Jump to navigation
Jump to search
A Clinical Data Interchange Standards Consortium (CDISC) Standard is a clinical data standard that was developed and maintained by the CDISC.
- Context:
- It can range from being a CDISC Foundational Standard, to being a CDISC Therapeutic Area (TA) Standard, to being a CDISC Transport Standard.
- …
- Example(s):
- Counter-Example(s):
- Digital Imaging and Communications in Medicine (DICOM) Standard,
- Health Level Seven (HL7) Standard,
- Logical Observation Identifiers Names and Codes (LOINC) Standard,
- OpenEHR,
- Systematized Nomenclature of Medicine (SNOMED) International Standard,
- United States Core Data for Interoperability (USCDI) Standard.
- See: Standard-Developing Organization (SDO), American National Standards Institute (ANSI), International Organization for Standardization (ISO), Clinical Trial Data, National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) Program, CDISC Shared Health And Research Electronic (SHARE) Library, Clinical Data Interchange Standards Consortium (CDISC) RWD Connect Initiative.
References
2022a
- (Facile et al., 2022) ⇒ Rhonda Facile, Erin Elizabeth Muhlbradt, Mengchun Gong, Qingna Li, Vaishali Popat, Frank Petavy, Ronald Cornet, Yaoping Ruan, Daisuke Koide, Toshiki I. Saito, Sam Hume, Frank Rockhold, Wenjun Bao, Sue Dubman, Barbara Jauregui Wurst (2022). "Use of Clinical Data Interchange Standards Consortium (CDISC) Standards for Real-world Data: Expert Perspectives From a Qualitative Delphi Survey". In: JMIR medical informatics, 10(1), e30363.
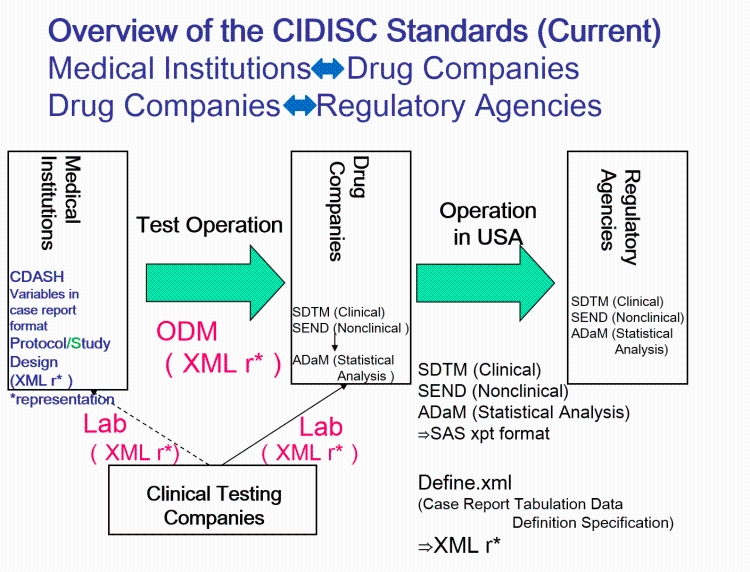
- QUOTE: The CDISC standards span the clinical research process and include standards for the exchange of nonclinical data (SEND), data collection case report forms (CRFs; clinical data acquisition standards harmonization (CDASH)), aggregation and tabulation (study data tabulation model (SDTM)), Biomedical Research Integrated Domain Group (BRIDG) logical model, and operational data model (ODM) for transport (Figure 1). In collaboration with the National Cancer Institute's Enterprise Vocabulary Services (NCI-EVS) program, CDISC has developed a rich controlled terminology that is linked to other common research semantics through the NCI-EVS tools. These standards, presented in data models, implementation guides, and user guides, are globally recognized and heavily used by the biopharmaceutical industry and some academic institutions.
2022b
- (HIMSS, 2022) ⇒ https://www.himss.org/resources/interoperability-healthcare#Part2 Retrieved:2022-2-26.
- QUOTE: There are over 40 different SDOs in the health IT arena. Some entities create standards, such as Health Level Seven (HL7), Systematized Nomenclature of Medicine (SNOMED) International, and the Clinical Data Interchange Standards Consortium (CDISC). Others, like Integrating the Healthcare Enterprise (IHE), do not develop new standards, but rather bundle complementary base standards into IHE profiles that are used to define a specific function or use case, and then are balloted. This creates a scenario that helps drive adoption of the base standards by providing implementation guidance that describes how multiple standards can be used together to support interoperable health information exchange.
2022c
- (Wikipedia, 2022) ⇒ https://en.wikipedia.org/wiki/Clinical_Data_Interchange_Standards_Consortium#Overview_of_standards Retrieved:2022-2-25.
- Dataset.XML (DataSet-XML)
- Enables communication of study results as well as regulatory submission to FDA (pilot since 2014).
- Study Data Tabulation Model (SDTM)
- Recommended for FDA regulatory submissions since 2004.
- The SDTM Implementation Guide (SDTM-IG) gives a standardized, predefined collection of domains for clinical data submission, each of which is based on the structure and metadata defined by the SDTM.
- Standard for Exchange of Non-clinical Data (SEND).
- The SEND Implementation Guide (SEND-IG) provides predefined domains and examples of non-clinical (animal) data based on the structure and metadata defined by the SDTM.
- Analysis Data Model (ADaM)
- Defines dataset and metadata standards that support statistical analyses and traceability. ADaM is one of the required standards for data submission to FDA (U.S.) and PMDA (Japan).
- Operational Data Model (ODM).
- The highlights of ODM: includes audit trail, utilizes XML technology, machine- and human- readable, all information are independent from databases, storing of ODM is independent from hard- and software.
- Laboratory Data Model (LAB)
- The Lab standard is used for exchange of laboratory data between labs and CROs
- Case Report Tabulation Data Definition Specification (CRT-DDS)
- Also referred to as “define.xml", a machine readable version of the regulatory submission "define.pdf".
- Clinical Data Acquisition Standards Harmonization (CDASH)
- Defines a minimal data collection set for sixteen safety SDTM Domains, harmonizing element names, definitions and metadata. The objective is to establish a standardized data collection baseline across all submissions.
- CDISC Terminology
- Defines controlled terminology for SDTM and CDASH, provides extensible lists of controlled terms designed to harmonize data collected across submissions.
- Dataset.XML (DataSet-XML)
2018
- (Hume et al., 2018) ⇒ Samuel Hume, Anthony Chow, Julie Evans, Frederik Malfait, Julie Chason, J. Darcy Wold, Wayne Kubick,and Lauren B. Becnel (2018). "CDISC SHARE, a Global, Cloud-based Resource of Machine-Readable CDISC Standards for Clinical and Translational Research". In: AMIA Summits on Translational Science Proceedings, 2018, 94.
- QUOTE: CDISC standards (Figure 1) are categorized as Foundational Standards, Semantics, Therapeutic Area (TA) Standards, and Transport Standards. Foundational Standards include Standard for Exchange of Nonclinical Data (SEND)[1] for the collection and tabulation of animal model and other pre-clinical data, Protocol Representation Model (PRM)[2], Analysis Data Model (ADaM)[3] for defining analysis datasets, Clinical Data Acquisition Standards Harmonization (CDASH)[4] that provide a minimal set of data elements common to essentially all studies, the Study Data Tabulation Model (SDTM)[5] for data tabulation, and others.

|
- ↑ Standard for Exchange of Nonclinical Data (SEND) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/send.
- ↑ CDISC. CDISC Protocol Representation Model Version 1.0. http://cdisc.org/ CDISC. 2010.
- ↑ Analysis Data Model (ADaM) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/adam
- ↑ Clinical Data Acquisition Standards Harmonization (CDASH) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/cdash.
- ↑ Study Data Tabulation Model (SDTM) Clinical Data Interchange Standards Consortium. 2017. Available from: https://www.cdisc.org/standards/foundational/sdtm.
2017
- (AMED, 2017) ⇒ https://www.amed.go.jp/en/aboutus/collaboration/cdisc.html Last updated 2017-4-7.
- QUOTE: The Clinical Data Interchange Standards Consortium (CDISC) develops and supports global and platform-independent data standards that enable information system interoperability to improve medical research and related areas of healthcare. It is an international interdisciplinary NPO established in 1997. In October 2016, the PMDA began accepting submissions of electronic clinical trial data conforming to CDISC standards by applicants of manufacture and marketing authorization of new drugs (this procedure was implemented by the FDA in previously years). In the future, conducting clinical trials including investigator initiated ones complied with to the CDISC standards will become necessary from the planning and implementation stages (...)