Adaptive Clinical Trial (ACT)
An Adaptive Clinical Trial (ACT) is a Clinical Trial that modifies parameters of the clinical trial protocol after observing clinical trial participant outcomes.
- Context:
- It can (typically) be conducted to evaluate a medical device or treatments after initial analysis of clinical endpoints.
- …
- Example(s):
- Adaptive COVID-19 Treatment Trial (ACTT),
- I-SPY 1 and 2 Clinical Trials,
- a Continual Reassessment Adaptive Clinical Trial such as:
- TRAFIC ACT (Cole et al., 2015),
- Viola ACT (Yap et al., 2017),
- RomiCar ACT, (Yap et al., 2013),
- a Group-Sequential and a Sample Size Re-Estimation ACT such as:
- DEVELOP-UK ACT (Fisher et al., 2016),
- a Multi-Arm Multi-Stage ACT such as:
- TAILoR ACT (Pushpakom et al., 2015),
- STAMPEDE ACT,
- COMPARE ACT,
- 18-F PET Study,
- a Population Enrichment ACT such as:
- a Biomarker-Adaptive Clinical Trial such as:
- an Adaptive Randomisation Clinical Trial such as:
- a Seamless ACT such as:
- an Adaptive Dose-Ranging Clinical Trial,
- …
- Counter-Example(s):
- See: Observational Clinical Trial, Confirmatory Clinical Trial, Explanatory Clinical Trial, Exploratory Clinical Trial, Pragmatic Clinical Trial, Decentralized Clinical Trial, Bandit Problem, Drug Development Clinical Trial, Medical Device Clinical Trial, Clinical Procedure Trial, Interventional Clinical Trial.
References
2021
- (Wikipedia, 2021) ⇒ https://en.wikipedia.org/wiki/Adaptive_clinical_trial Retrieved:2021-11-13.
- An adaptive clinical trial is a clinical trial that evaluates a medical device or treatment by observing participant outcomes (and possibly other measures, such as side-effects) on a prescribed schedule, and modifying parameters of the trial protocol in accord with those observations. The adaptation process generally continues throughout the trial, as prescribed in the trial protocol. Modifications may include dosage, sample size, drug undergoing trial, patient selection criteria and "cocktail" mix. In some cases, trials have become an ongoing process that regularly adds and drops therapies and patient groups as more information is gained.[1] Importantly, the trial protocol is set before the trial begins; the protocol pre-specifies the adaptation schedule and processes.
The aim of an adaptive trial is to more quickly identify drugs or devices that have a therapeutic effect, and to zero in on patient populations for whom the drug is appropriate.[2] A key modification is to adjust dosing levels. Traditionally, non-adverse patient reactions are not considered until a trial is completed. [3]
The problem of adaptive clinical trial design is more or less exactly the bandit problem as studied in the field of reinforcement learning.
- An adaptive clinical trial is a clinical trial that evaluates a medical device or treatment by observing participant outcomes (and possibly other measures, such as side-effects) on a prescribed schedule, and modifying parameters of the trial protocol in accord with those observations. The adaptation process generally continues throughout the trial, as prescribed in the trial protocol. Modifications may include dosage, sample size, drug undergoing trial, patient selection criteria and "cocktail" mix. In some cases, trials have become an ongoing process that regularly adds and drops therapies and patient groups as more information is gained.[1] Importantly, the trial protocol is set before the trial begins; the protocol pre-specifies the adaptation schedule and processes.
- ↑ Brennan, Zachary (2013-06-05). "CROs Slowly Shifting to Adaptive Clinical Trial Designs". Outsourcing-pharma.com. Retrieved 2014-01-05.
- ↑ Wang, Shirley S. (2013-12-30). "Health: Scientists Look to Improve Cost and Time of Drug Trials - WSJ.com". Online.wsj.com. Archived from the original on 2016-03-14. Retrieved 2014-01-04.
- ↑ Peter W. Huber (12 November 2013). The Cure in the Code: How 20th Century Law Is Undermining 21st Century Medicine. Basic Books. ISBN 978-0-465-06981-1.
2019
- (FDA, 2019) ⇒ "Adaptive Designs for Clinical Trials of Drugs and Biologics. Guidance for Industry".
- QUOTE: In general, the design, conduct, and analysis of an adaptive clinical trial intended to provide substantial evidence of effectiveness should satisfy four key principles: the chance of erroneous conclusions should be adequately controlled, estimation of treatment effects should be sufficiently reliable, details of the design should be completely prespecified, and trial integrity should be appropriately maintained. While all clinical trials intended to provide substantial evidence of effectiveness should satisfy these four principles, the following sections outline considerations specific to adaptive designs.
2018
- (Pallmann et al., 2018) ⇒ Philip Pallmann, Alun W. Bedding, Babak Choodari-Oskooei, Munyaradzi Dimairo, Laura Flight, Lisa V. Hampson, Jane Holmes, Adrian P. Mander, Lang’o Odondi, Matthew R. Sydes, Sofia S. Villar, James M. S. Wason, Christopher J. Weir, Graham M. Wheeler, Christina Yap, and Thomas Jaki (2018). "Adaptive Designs in Clinical Trials: Why Use Them, and How to Run and Report Them". In: BMC medicine, 16(1), 1-15.
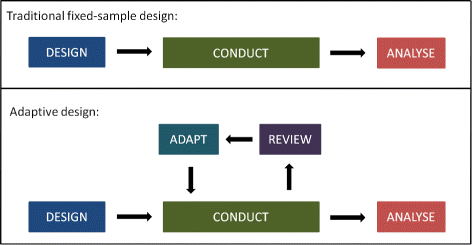
- QUOTE: Adaptive designs can make clinical trials more flexible by utilising results accumulating in the trial to modify the trial’s course in accordance with pre-specified rules. Trials with an adaptive design are often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money, and might require fewer participants(...)
Pre-planned changes that an AD may permit include, but are not limited to (...):
- refining the sample size;
- abandoning treatments or doses.
- changing the allocation ratio of patients to trial arms.
- identifying patients most likely to benefit and focusing recruitment efforts on them
- stopping the whole trial at an early stage for success or lack of efficacy.
- QUOTE: Adaptive designs can make clinical trials more flexible by utilising results accumulating in the trial to modify the trial’s course in accordance with pre-specified rules. Trials with an adaptive design are often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money, and might require fewer participants(...)
- Table 1 lists some well-recognised adaptations and examples of their use. Note that multiple adaptations may be used in a single trial, e.g. a group-sequential design may also feature mid-course sample size re-estimation and/or adaptive randomisation (...), and many multi-arm multi-stage (MAMS) designs are inherently seamless (...). ADs can improve trials across all phases of clinical development, and seamless designs allow for a more rapid transition between phases I and II (...) or phases II and III (...).
[
|
2016
- (Bhatt & Mehta, 2016) ⇒ Deepak L. Bhatt, and Cyrus Mehta (2016). "Adaptive Designs for Clinical Trials". In: The New England Journal of Medicine (NEJM), 2016; 375:65-74. DOI: 10.1056/NEJMra1510061.
- QUOTE: Adaptive designs are applicable to both exploratory and confirmatory clinical trials. Adaptive designs for exploratory clinical trials deal mainly with finding safe and effective doses or with dose–response modeling. The emphasis is on strategies that will assign a larger proportion of the participants to treatment groups that are performing well, reduce the number of participants in treatment groups that are performing poorly, and investigate a dose range that is larger than ranges in corresponding trials with nonadaptive designs, in order to select effective doses for the confirmatory stage of investigation. Control of the type I error rate is less of an issue. In Table 1, various types of adaptive designs for exploratory clinical trials are classified into categories that reflect the time sequence in which they would be performed in the drug-development process.
2014
- (Chow, 2014) ⇒ Shein-Chung Chow. (2014). “Adaptive Clinical Trial Design.” In: Annual Review of Medicine, 65.
- There is no universal definition: Adaptive randomization, group sequential, and sample size re-estimation, etc. (Chow, Chang and Pong, 2005), (PhRMA, 2006)
- Adaptive design is also known as: Flexible design (EMEA, 2002, 2006), Attractive design (Uchida, 2006)
- Characteristics:
- Adaptation is a design feature.
- Changes are made “by design” not on an “ad hoc” basis.
- Comments
- It does not reflect real practice.
- It may not be flexible as it means to be.
2010
- (Mahajan & Gupta) ⇒ Rajiv Mahajan, and Kapil Gupta (2010). "Adaptive Design Clinical Trials: Methodology, Challenges and Prospect". In: Indian Journal of Pharmacology, 42(4).
- QUOTE: An adaptive design is defined as a design that allows modifications to the trial and/or statistical procedures of the trial after its initiation without undermining its validity and integrity (...). The purpose is to make clinical trials more flexible, efficient and fast. Due to the level of flexibility involved, these trial designs are also termed as “flexible designs”(...)
Based on adaptations employed, commonly considered adaptive design methods in clinical trials include an adaptive randomization design, a group sequential design, a sample size re-estimation design, a drop-the-loser design, an adaptive dose finding design, a biomarker-adaptive design, an adaptive treatment-switching design, a hypothesis-adaptive design, an adaptive seamless phase II/III trial design and a multiple adaptive design.
- QUOTE: An adaptive design is defined as a design that allows modifications to the trial and/or statistical procedures of the trial after its initiation without undermining its validity and integrity (...). The purpose is to make clinical trials more flexible, efficient and fast. Due to the level of flexibility involved, these trial designs are also termed as “flexible designs”(...)