Clinical Research Study
(Redirected from Clinical Research Project)
Jump to navigation
Jump to search
A Clinical Research Study is a designed empirical study that seeks to understand clinical outcomes (of clinical investigational products) on clinical study participants.
- Context:
- Input: Experimental Therapy(?).
- Output: Clinical Research Study Data; Clinical Research Study Report.
- It can (typically) be associated with a Clinical Study Process.
- It can (typically) be composed of Clinical Study Subtasks, such as clinical study participant recruitment.
- It can (typically) be associated with a Clinical Study Status, such as terminated clinical study.
- It can (typically) be described in a Clinical Study Plan Document.
- It can (typically) involve Acts of Clinical Research Consent.
- It can be a part of a Clinical Product Evaluation Process (such as a clinical drug evaluation process).
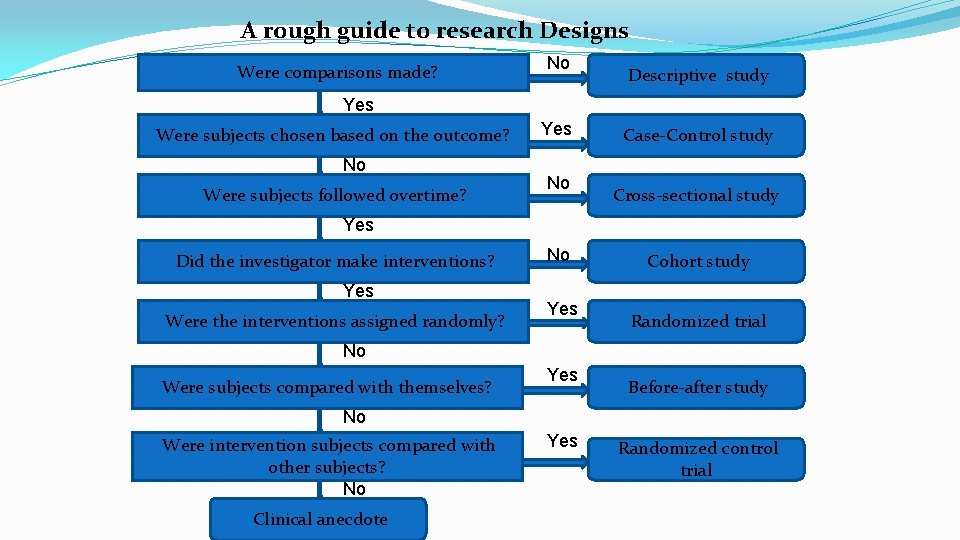
- It can range from being a Interventional Clinical Research Study to being a Observational Clinical Research Study.
- It can range from being a Clinical Drug Research Study to being a Clinical Device Research Study.
- It can range from being a Envisioned Clinical Study to being an Active Clinical Study to being a Inactive Clinical Study (such as a concluded clinical study).
- It can result in a Clinical Research Study Analysis.
- It can support Evidence-based Medicine.
- It can (typically) involve support from Cliical Research Study Personas, such as a clinical study participant, clinical study principal investigator, and clinical study site monitor.
- …
- Example(s):
- a Clinical Research Intervention Study, such as:
- a Randomized Clinical Trial Research Study, were intervention subjects compared with other subjects.
- …
- a Clinical Research Observational Study, such as:
- …
- a Clinical Research Intervention Study, such as:
- Counter-Example(s):
- See: Clinical Study Standard, Good Clinical Practice, Clinical Research Meta Analysis.
References
2020
- https://clinicaltrials.gov/ct2/about-studies/glossary
- QUOTE: Clinical study: A research study involving human volunteers (also called participants) that is intended to add to medical knowledge. There are two types of clinical studies: interventional studies (also called clinical trials) and observational studies.
2020
- https://clinicaltrials.gov/ct2/about-studies/learn#ReasonsForConducting
- QUOTE: ... In general, clinical studies are designed to add to medical knowledge related to the treatment, diagnosis, and prevention of diseases or conditions. Some common reasons for conducting clinical studies include:
- Evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition
- Finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches.
- Evaluating one or more interventions aimed at identifying or diagnosing a particular disease or condition
- Examining methods for identifying a condition or the risk factors for that condition
- Exploring and measuring ways to improve the comfort and quality of life through supportive care for people with a chronic illness
- QUOTE: ... In general, clinical studies are designed to add to medical knowledge related to the treatment, diagnosis, and prevention of diseases or conditions. Some common reasons for conducting clinical studies include:
2016
- (Krleža-Jerić et al, 2016) ⇒ Karmela Krleža-Jerić, Mirko Gabelica, Rita Banzi, Marina Krnić-Martinić, Bibiana Pulido, Mersiha Mahmić-Kaknjo, Ludovic Reveiz, Josip Šimić, Ana Utrobičić, and Irena Hrgović. “IMPACT Observatory: tracking the evolution of clinical trial data sharing and research integrity." In: Biochemia Medica, 26(3).
- ABSTRACT: ... The opening of research data is emerging thanks to the increasing possibilities of digital technology. The opening of clinical trial (CT) data is a part of this process, expected to have positive scientific, ethical, health, and economic impacts thus contributing to research integrity. ...
- QUOTE:

Figure 1 Evidence pyramid. The hierarchy of evidence and the role of the individual participant data (IPD) meta-analysis in knowledge creation is presented. The reliability of evidence needed for evidence-informed decision-making in health increases as we move up the pyramid. It is expected that IPD meta-analysis would speed the knowledge creation.